Digital Medical Ophthalmoscope (DMO): A Comprehensive Guide for Biomedical Engineers and Healthcare Professionals
Table of Contents
- What is a Digital Medical Ophthalmoscope (DMO)
- A Short History
- How It Works: The Imaging Principle
- Key Hardware Components
- Clinical Applications & Use Cases
- DMO vs Other Ophthalmic Imaging Devices
- Safety & Patient Comfort Considerations
- Maintenance & Technical Considerations (for Biomed/CE Teams)
- Cost & Accessibility
- Future Innovations & Trends
- Simple Analogies to Demystify DMO
- Final Summary
- References
What is a Digital Medical Ophthalmoscope (DMO)
A Digital Medical Ophthalmoscope (DMO) is an advanced diagnostic imaging device used to visualize and capture detailed images of the retina, optic disc, and posterior segment of the eye.
Unlike traditional optical ophthalmoscopes that rely purely on direct visual observation, DMOs use digital sensors and optical systems to produce high-resolution digital images or video streams that can be stored, analyzed, and transmitted electronically.
DMOs play a crucial role in the early detection and management of retinal diseases, including diabetic retinopathy, glaucoma, and macular degeneration.

A Short History of DMO
- 1851: Hermann von Helmholtz invented the first ophthalmoscope, revolutionizing ophthalmic diagnostics.
- 1960s–1980s: Introduction of indirect ophthalmoscopes and fundus cameras for clinical photography.
- 1990s–2000s: Integration of CCD/CMOS sensors enabled digital fundus imaging.
- Today: Portable handheld DMOs and smartphone-based models offer teleophthalmology capabilities, enabling remote retinal screening in rural and low-resource settings.
How It Works: The Imaging Principle
The DMO operates on the principle of indirect ophthalmoscopy combined with digital image capture:
- A low-intensity light source (LED or halogen) illuminates the retina through the pupil.
- Reflected light passes through optical lenses that focus the retinal image onto a digital image sensor (CCD or CMOS).
- The captured image is processed using onboard or external software for enhancement, annotation, and storage.
- Images can be transmitted via DICOM or telemedicine platforms for review and consultation.
Analogy: Think of the retina as the “film” in a camera. The ophthalmoscope acts as both the light and lens system, while the digital sensor serves as the camera sensor, recording everything in fine detail.

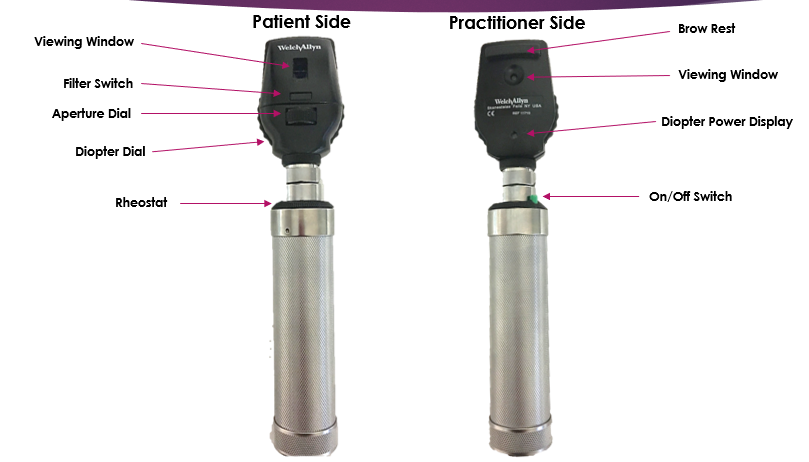
Key Hardware Components
- Optical System: Lens assembly to magnify and focus the retinal image.
- Light Source: LED or halogen illumination; infrared (IR) for non-mydriatic imaging.
- Digital Sensor: High-resolution CCD/CMOS for image capture.
- Display & Interface: Built-in screen or tablet for viewing; some systems connect wirelessly to PCs or cloud platforms.
- Power Supply: Rechargeable batteries (portable) or mains power (desktop).
- Software Platform: Image enhancement, measurement tools, AI-based diagnostic assistance, and DICOM export.
Clinical Applications & Use Cases
- Diabetic Retinopathy Screening
- Glaucoma Monitoring (optic nerve evaluation)
- Age-related Macular Degeneration (AMD)
- Retinal Detachment Assessment
- Teleophthalmology & Remote Screening
- Documentation of Ocular Findings in EMR systems
DMOs have become essential not only in hospitals but also in primary care clinics and telemedicine programs, bridging the gap between screening and specialist care.
DMO vs Other Ophthalmic Imaging Devices
| Feature | Digital Ophthalmoscope (DMO) | Fundus Camera | OCT (Optical Coherence Tomography) | Slit Lamp with Camera |
|---|---|---|---|---|
| Imaging Principle | Optical + digital reflection | Optical photography | Optical interferometry | Visible light microscopy |
| Field of View | 20–55° | 30–90° | Limited cross-section | Variable |
| Depth Resolution | Moderate | Moderate | Very High (micron-level) | Low |
| Use Case | General retinal screening | Detailed fundus photography | Retinal layer analysis | Anterior/posterior visualization |
| Portability | High | Low–Medium | Low | Low |
| Cost Range | $3K–$15K | $15K–$50K | $60K–$150K | $10K–$25K |
Safety & Patient Comfort Considerations
- Non-invasive and painless.
- Low-intensity light minimizes retinal stress.
- Non-mydriatic models allow imaging without pupil dilation, improving patient comfort.
- Adherence to IEC/ISO photobiological safety standards is essential.
Maintenance & Technical Considerations (for Biomed/CE Teams)
- Optical alignment: Regular calibration for focus accuracy.
- Sensor cleaning: Avoid dust or fingerprints on the sensor/lens.
- Firmware/software updates: Ensure compatibility with hospital PACS.
- Battery maintenance: Replace after 300–500 charge cycles for handheld units.
- QC protocol: Use calibration targets for color and brightness validation.
Cost & Accessibility
| Category | Approx. Price (USD) | Example Systems |
|---|---|---|
| Handheld Non-Mydriatic | $3,000 – $7,000 | Volk Phelcom Eyer, Optomed Aurora |
| Tabletop Digital Fundus | $8,000 – $20,000 | Topcon NW400, Canon CR-2 AF |
| High-End AI-Integrated DMO | $20,000 – $50,000 | Zeiss Visucam 524, Remidio Fundus on Phone AI |
Accessibility has improved significantly thanks to portable and smartphone-based DMOs, which are vital in community screening and developing regions.
Future Innovations & Trends
- AI Integration: Automated diabetic retinopathy detection (FDA-approved systems like IDx-DR).
- Teleophthalmology Platforms: Seamless cloud connectivity for remote diagnosis.
- Photon-Counting Sensors: Promising better dynamic range and reduced noise.
- 3D Retinal Reconstruction: Hybrid DMO-OCT systems under research.
- Ergonomic Design: Lighter handheld models and wireless charging.
Simple Analogies to Demystify DMO
- DMO vs Traditional Ophthalmoscope: Like switching from a flashlight and mirror to a digital camera with autofocus.
- Non-mydriatic Imaging: Like taking a photo in a dim room without forcing the subject to open their eyes wider.
- AI-Assisted Screening: Similar to having a second expert reviewing every image instantly.
Final Summary
- DMOs bring precision, portability, and digital connectivity to eye diagnostics.
- They are vital for early detection of retinal diseases and telemedicine.
- Biomedical engineers should focus on optical alignment, sensor maintenance, and calibration.
- Future devices will integrate AI, cloud platforms, and 3D imaging.
- The technology is becoming more affordable and accessible, expanding vision care globally.
References
- World Health Organization (WHO) – Vision 2020: The Right to Sight Initiative.
- Silva PS et al. Teleophthalmology and diabetic retinopathy screening. Diabetes Care, 2021.
- IDx Technologies. FDA Approval for Autonomous AI-based Retinopathy Detection.
- Zeiss, Canon, Topcon, Optomed — Manufacturer Technical Datasheets.
- American Academy of Ophthalmology (AAO). Clinical Guidelines for Retinal Imaging.
