How Biomedical Devices Are Classified: Insights into FDA Classification
In the rapidly evolving field of biomedical engineering, understanding the regulatory landscape is crucial for the successful development and deployment of medical devices. The U.S. Food and Drug Administration (FDA) plays a pivotal role in this process by classifying medical devices to ensure their safety and effectiveness. This article provides a comprehensive overview of how biomedical devices are classified, with a focus on the FDA’s classification panels and the criteria used to determine device classes.
1. Introduction to FDA Medical Device Classification
The FDA categorizes medical devices to establish the level of regulatory control necessary to ensure their safety and effectiveness. This classification system helps manufacturers understand the requirements for marketing their devices in the United States.
Importance of Device Classification:
- Regulatory Compliance: Determines the necessary premarket approval or clearance processes.
- Safety Assurance: Ensures that devices meet specific standards to protect public health.
- Market Access: Facilitates the introduction of new devices into the healthcare system.
2. Understanding FDA’s Classification System
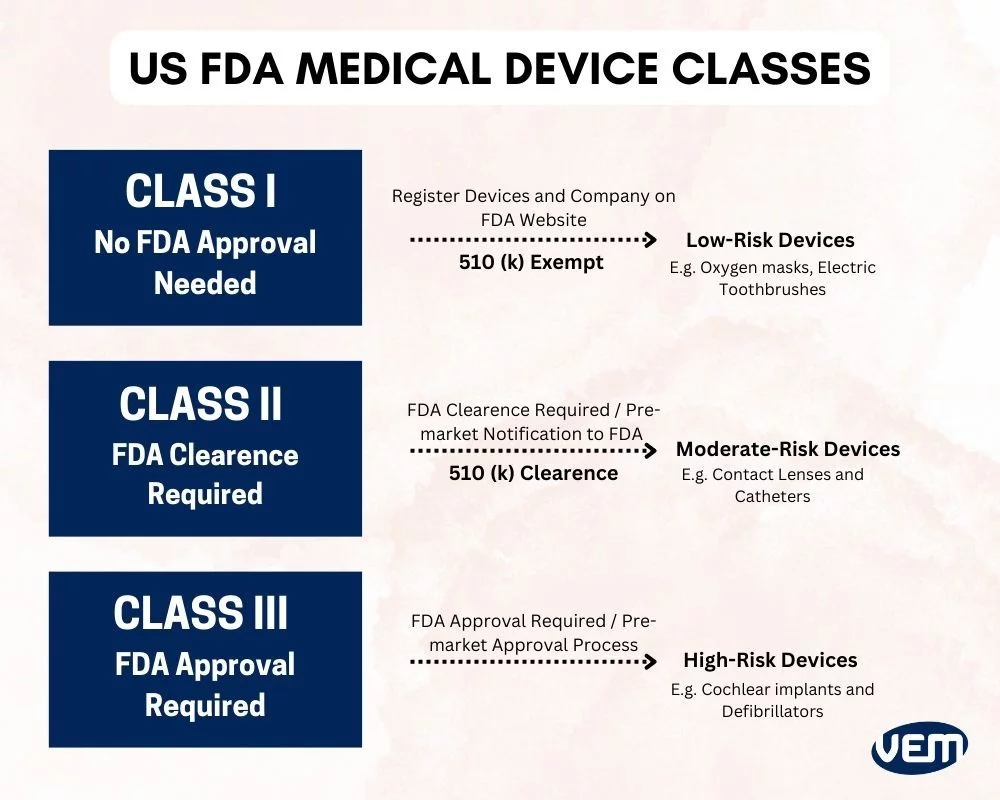
The FDA classifies medical devices into three primary classes:
Class I: General Controls
- Description: Devices with low to moderate risk.
- Regulatory Controls: Subject to general controls, including provisions related to adulteration, misbranding, device registration, and good manufacturing practices.
- Examples: Elastic bandages, examination gloves.
Class II: General Controls and Special Controls
- Description: Devices with moderate to high risk.
- Regulatory Controls: Subject to general controls and special controls such as performance standards, postmarket surveillance, and patient registries.
- Examples: Powered wheelchairs, infusion pumps.
Class III: General Controls and Premarket Approval
- Description: Devices with high risk, usually those that support or sustain human life, are of substantial importance in preventing impairment of human health, or present a potential unreasonable risk of illness or injury.
- Regulatory Controls: Subject to general controls and require premarket approval (PMA) to ensure safety and effectiveness.
- Examples: Implantable pacemakers, heart valves.
This classification framework is detailed in Title 21 of the Code of Federal Regulations (CFR), Parts 862-892.
3. Overview of FDA Classification Panels
The FDA has established 16 medical specialty panels, each responsible for a specific category of devices. These panels are outlined in Title 21 of the Code of Federal Regulations (CFR), Parts 862-892. Each panel encompasses a range of devices related to a particular medical specialty.
List of FDA Classification Panels:
- Anesthesiology (Part 868): Devices related to anesthesia and respiratory functions.
- Cardiovascular (Part 870): Devices associated with heart and circulatory system functions.
- Chemistry (Part 862): Devices for clinical chemistry and in vitro diagnostic use.
- Dental (Part 872): Devices used in dental practice.
- Ear, Nose, and Throat (Part 874): Devices for otolaryngological procedures.
- Gastroenterology and Urology (Part 876): Devices pertaining to the digestive and urinary systems.
- General and Plastic Surgery (Part 878): Devices for general surgical and aesthetic procedures.
- General Hospital (Part 880): Devices commonly used in hospital settings.
- Hematology (Part 864): Devices related to blood and blood-forming tissues.
- Immunology (Part 866): Devices for immunological testing.
- Microbiology (Part 866): Devices for microbiological testing.
- Neurology (Part 882): Devices associated with neurological functions.
- Obstetrical and Gynecological (Part 884): Devices for reproductive health and childbirth.
- Ophthalmic (Part 886): Devices related to eye care and vision correction.
- Orthopedic (Part 888): Devices for musculoskeletal system support and repair.
- Pathology (Part 864): Devices used in pathological examinations.
- Physical Medicine (Part 890): Devices for physical therapy and rehabilitation.
- Radiology (Part 892): Devices involving medical imaging and radiation therapy.
- Toxicology (Part 862): Devices for clinical toxicology.
4. Determining Device Classification
To determine a device’s classification, manufacturers should:
- Identify the Device’s Intended Use: Clearly define what the device is designed to do.
- Consult the CFR: Review Parts 862-892 to find a matching device description.
- Assess Risk Level: Evaluate the potential risk the device poses to patients and users.
This process helps in identifying the appropriate classification and the regulatory requirements that apply.
5. Regulatory Controls Associated with Each Class
Each device class has specific regulatory controls:
- Class I – General Controls:
- Establishment registration
- Device listing
- Good Manufacturing Practices (GMP)
- Labeling requirements
- Class II – General and Special Controls:
- Includes all Class I controls
- Special controls like performance standards
- Postmarket surveillance
- Patient registries
- Class III – Premarket Approval (PMA):
- Requires scientific evidence demonstrating safety and effectiveness
- Rigorous review process
These controls are designed to provide reasonable assurance of the device’s safety and effectiveness.
6. Pathways to Market: 510(k) and PMA
Depending on the device classification, there are two primary pathways to bring a device to market:
- 510(k) Premarket Notification: Required for most Class II devices. Manufacturers must demonstrate that their device is substantially equivalent to a legally marketed predicate device.
- Premarket Approval (PMA): Required for Class III devices. This involves a more rigorous process, including clinical trials, to provide valid scientific evidence of the device’s safety and effectiveness.
Understanding these pathways is crucial for compliance and successful market entry.
7. Conclusion
Understanding the FDA’s classification system for biomedical devices is essential for ensuring their safety and effectiveness. By categorizing devices into Class I, II, or III based on risk, the FDA provides a structured framework that guides manufacturers, healthcare professionals, and patients. This system not only facilitates innovation but also upholds public health standards. Staying informed about these classifications enables stakeholders to navigate regulatory pathways more efficiently, ensuring that medical devices meet the necessary requirements for market approval and clinical use.
7. Refereneces
https://www.fda.gov/medical-devices/classify-your-medical-device/device-classification-panels. (n.d.).
SciSpace: The fastest research platform ever designed, All-in-one AI tools for students and researchers. https://www.typeset.io/?via=biomedevices
